What Is A Bohr Diagram Hanenhuusholli
2. What elements is represented by this diagram? How do you know? 3. What is the charge of this nucleus? What is the charge of this atom overall? 4. What is the mass of this atom? 5. Using a periodic table, look up titanium. a. What is its atomic number? b. How many protons does a titanium atom have? c. How many electrons does it have? 6. What.
Symbol and electron diagram for silicon Royalty Free Vector
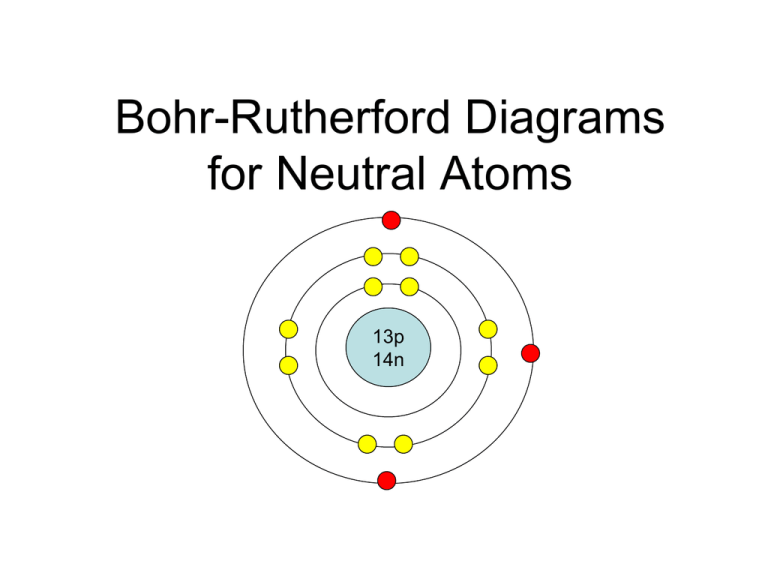
draw a Bohr-Rutherford diagram for sulfur. draw a Bohr-Rutherford diagram for chlorine. draw a Bohr-Rutherford diagram for argon. draw a Bohr-Rutherford diagram for potassium. draw a Bohr-Rutherford diagram for calcium. Identification of elements from Bohr-Rutherford Diagram. Learn with flashcards, games, and more — for free.
Bohr Model For Silicon
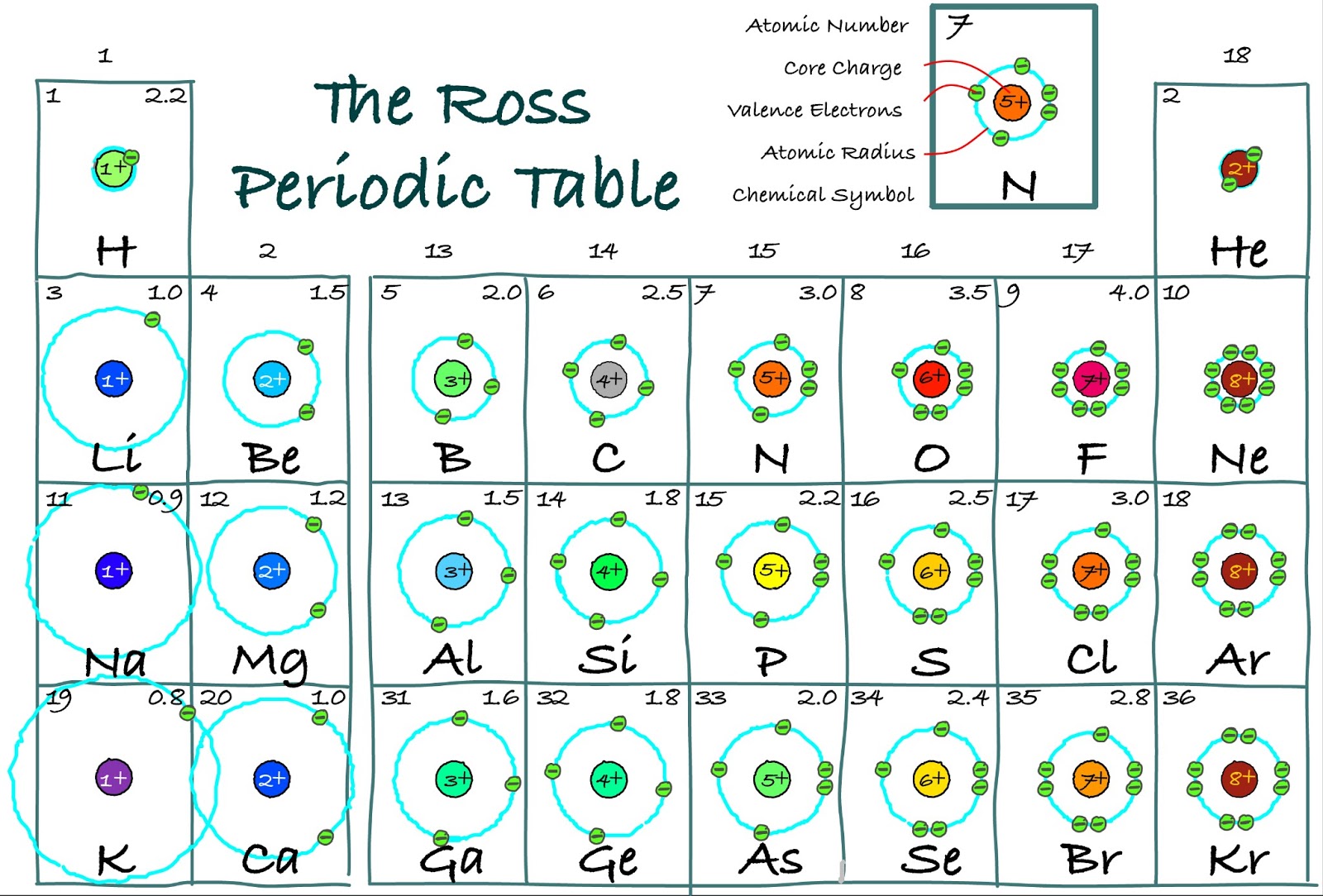
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
Silicon Atomic Number Bohr Model Chemical Element, PNG, 1000x1000px
4) The following diagram is a Bohr-Rutherford diagram of one element from the periodic table: To which group and period does this element belong? A) Period 3 group 4. B) Period 4 group 4. C) Period 3 group 1. D) Period 1 group 3. 5) Referring to the periodic table, find an element that has the same number of electron shells and
FileThis shows the bond of Silicon Oxide using the Bohr model.png
Mr. Primmer Demonstrates How to Draw Bohr Rutherford Diagrams!
Bohr model of silicon atom Electronics And Engineering Lab
This page contains materials for the session on the atomic models of Rutherford and Bohr. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study.

View 19 Silicon Element Bohr Model aboutdiecolor
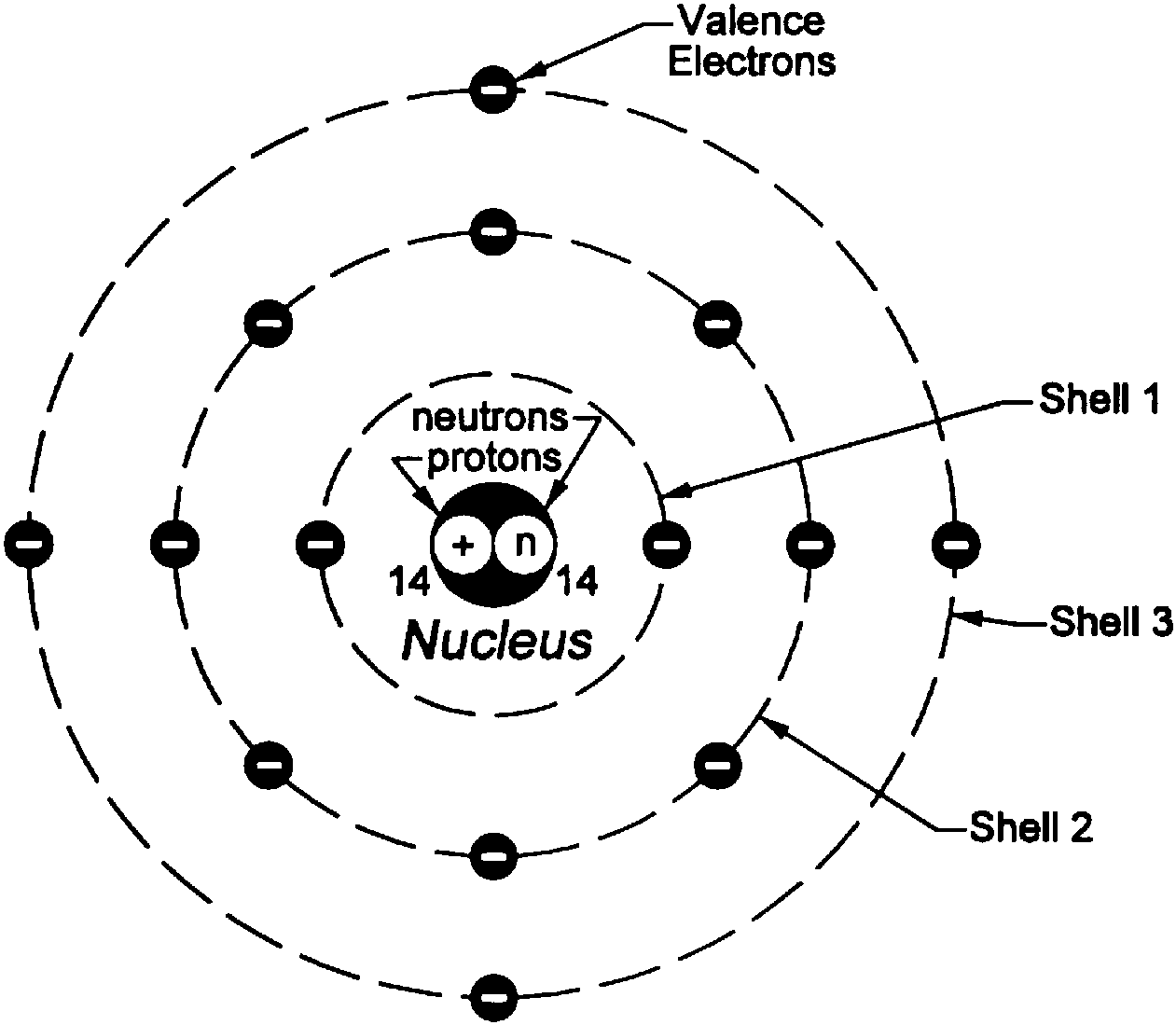
In atomic physics, the Bohr model or Rutherford-Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons.
Electron arrangements
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons.

Image result for silicon atomic model
The Bohr Model has an atom consisting of a small, positively charged nucleus orbited by negatively charged electrons. Here's a closer look at the Bohr Model, which is sometimes called the Rutherford-Bohr Model. Overview of the Bohr Model Niels Bohr proposed the Bohr Model of the Atom in 1915.
Bohr Model Silicon Atom Electron Structure Stock Vector (Royalty Free
sorry for my stutter, i stutter when i talk fast & sorry for any delay that occuredthe website i used is called webwhiteboard.com
Silicon Si (Element 14) of Periodic Table Elements FlashCards
Manish Bhardwaj. 5.6: Bohr Diagrams of Atoms and Ions is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells,..
valence electrons of silicon
Contents show Bohr Model of Silicon The Bohr model is also known as the Bohr-Rutherford model as it was developed as a modification of the Rutherford model. It was given by Niel Bohr in 1913. This model is used to illustrate the atomic composition in pictorial form.
_0.jpg?itok=RuBFwyf1)
Le modèle atomique de RutherfordBohr Alloprof
Bohr diagram or Bohr rutherford diagram describes the visual representation of orbiting electrons around the small nucleus. It used different electron shells such as K, L, M, N…so on.
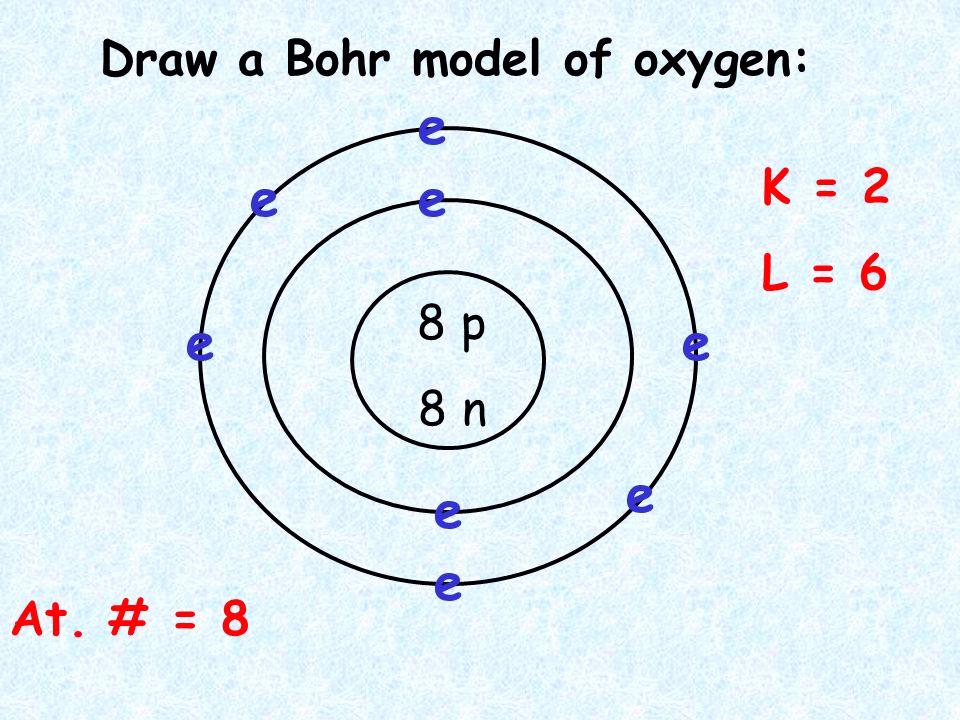
Diagram Bohr Model Periodic Table Periodic Table Timeline
In this video we'll look at the atomic structure and Bohr model for the Silicon atom (Si). We'll use a Bohr diagram to visually represent where the electrons.

Number of valence electrons in silicon
Draw a molecule of SiS 2, using Bohr-Rutherford diagrams showing all electrons (core electrons and valence electrons). Ensure that all atoms in the molecule have a full valence shell. Show your work. Draw a molecule of SiS 2 as a Lewis dot diagram, showing all valence electrons; Answer the following questions about the molecule you drew in.
BohrRutherford diagrams for atoms
The drawbacks of the Rutherford model of the atom, including its apparent instability, would soon be addressed by his student, Dutch physicist Neils Bohr (1885 - 1962). To unlock this lesson you.