
ScIU Conversations in Science at Indiana University
In 1913, a Danish physicist, Niels Bohr (1885-1962; Nobel Prize in Physics, 1922), proposed a theoretical model for the hydrogen atom that explained its emission spectrum. Bohr's model required only one assumption: The electron moves around the nucleus in circular orbits that can have only certain allowed radii.

Sodium Bohr Diagram
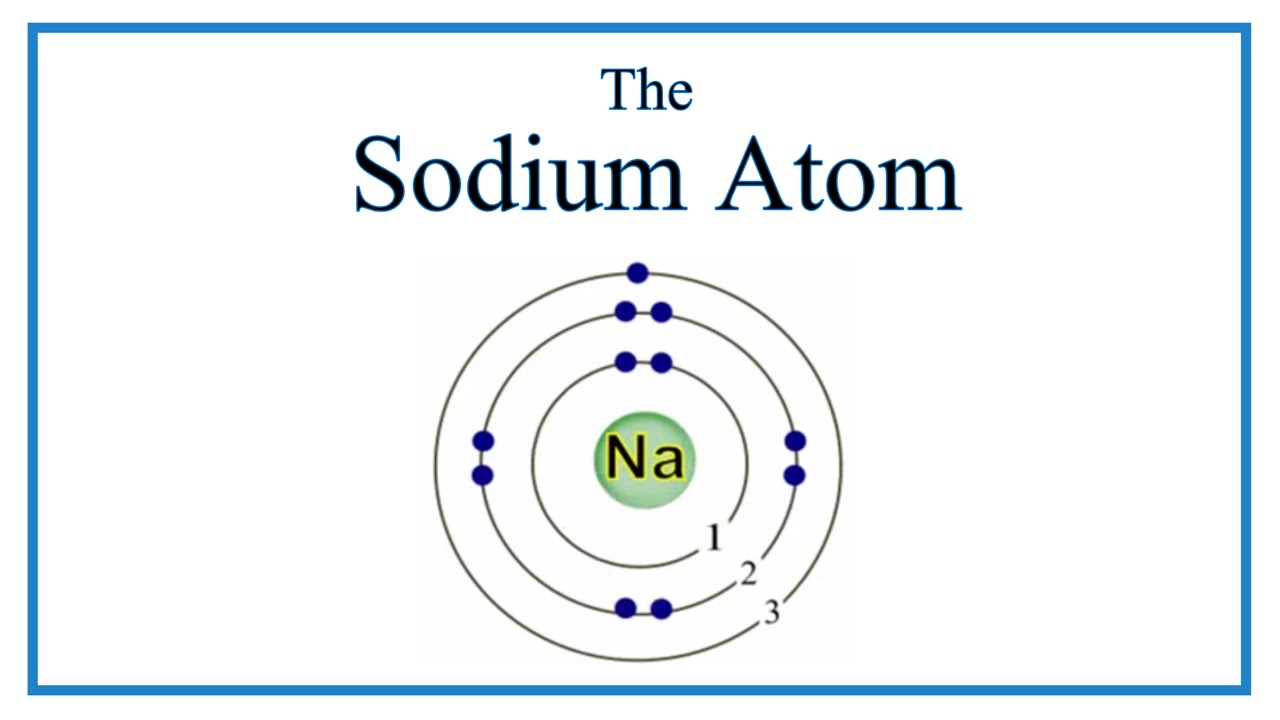
The Bohr Model of Sodium (Na) has a nucleus that contains 12 neutrons and 11 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sodium contains only 1 electron that also called valence electron. Page Contents show How to draw Bohr Model of Sodium (Na)?
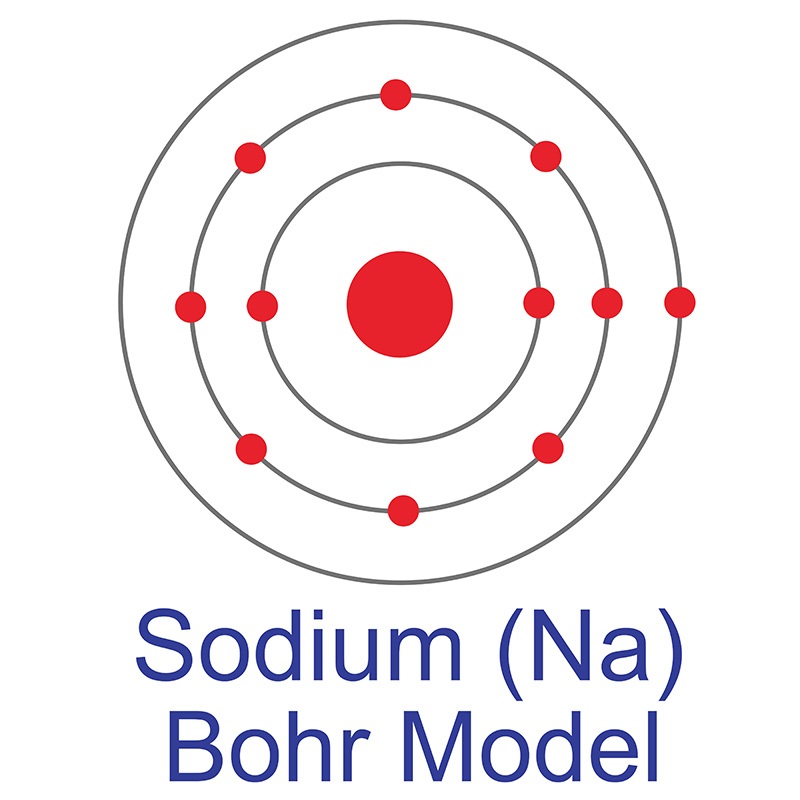
Sodium (Na) AMERICAN ELEMENTS
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.
Bohr Model of Sodium Science ShowMe
Key points Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, n : E ( n) = − 1 n 2 ⋅ 13.6 eV

Figure \ Electron Shell Configuration Of Sodium 1200x1200 PNG
The electron cloud model An electron cloud model of a helium-4 atom is shown below. [What do the scales mean on this model?] In this model, the black "cloud" represents the volume of space where electrons are likely to be found. The darker the region, the more likely electrons are to be found there.
Sodium Facts & Bohr Model YouTube
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.

Sodium Bohr model
Figure \(\PageIndex{1}\): A Bhor's model can be used to diagram the location of electrons in each energy shell for an atom. Notice that protons go in the nucleus of the atom and electrons are drawn on orbits surrounding the nucleus. Image from Wikimedia commons. Example \(\PageIndex{2}\) Draw the Bohr's model for sodium (Na).
Bohr Model Of A Phosphorus Atom Electron Configuration Of Sodium
which is identical to the Rydberg equation in which R ∞ = k h c. R ∞ = k h c. When Bohr calculated his theoretical value for the Rydberg constant, R ∞, R ∞, and compared it with the experimentally accepted value, he got excellent agreement. Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that.
Bohr Model Of Sodium
Thanks Erik T. for being so awesome at chemistry and willing to teach us how to build a sodium atom.-----Thanks for watching and please subscribe for more sc.
.PNG)
Bohr Model Of Sodium
Bohr's Model of an Atom was proposed in 1913 as a modification of the prevailing Saturnian model developed by Bohr's mentor Ernest Rutherford, and the Bohr model is sometimes referred to.

Bohr Diagram Of Sodium
Creating a sodium Bohr diagram is a way to visually represent the arrangement of electrons in a sodium atom based on the Bohr model. The Bohr model suggests that electrons occupy specific energy levels or shells around the nucleus of an atom. Each shell can hold a certain number of electrons, and the diagram helps to show the distribution of.

Atom illustration, Bohr model Sodium Atom Chemistry Rutherford model
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.
.PNG)
Bohr Models and Lewis Dot Diagrams Presentation Chemistry
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).
Bohr Model Drawing Oxygen at Explore collection of
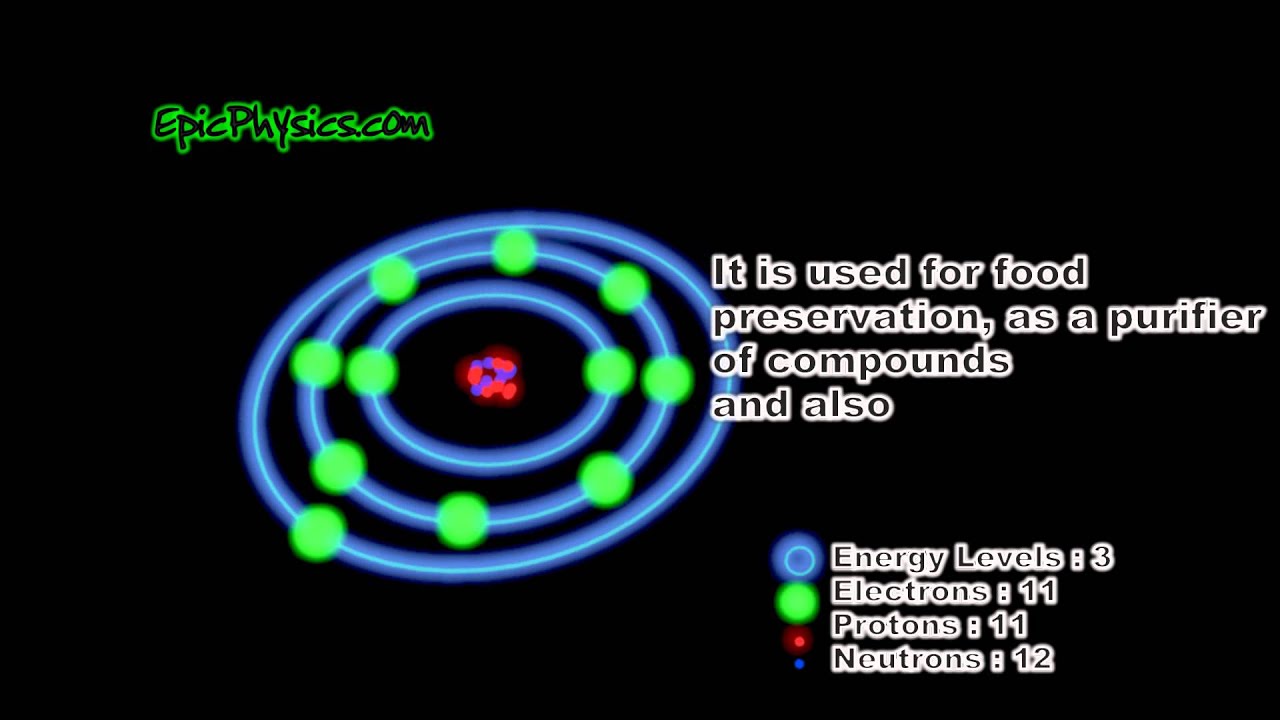
The Bohr model of sodium contains a nucleus having 11 protons and 12 neutrons in the center, and around this nucleus, there are three electron shells containing 11 electrons. Atomic Structure of the Sodium Atom (Na) Watch on Contents Steps #1 Write protons, neutrons, and electrons of sodium atom #2 Draw nucleus of sodium atom

Sodium Bohr Model — Diagram, Steps To Draw Techiescientist
Immediately before 1913, the Rutherford model conceived of an atom as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii. Britannica Quiz Matter and More Quiz How does Niels Bohr's atomic model work?

FileElectron shell 011 sodium.png Wikimedia Commons
The Bohr Model is a modification of an earlier atomic model, the Rutherford Model. The Bohr Model has an atom with a positively-charged nucleus surrounded by negatively-charged electrons that have circular, planetary-like orbits. Today, we know that the Bohr Model has some inaccuracies, but it's still used because of its simple approach to.